Case Studies in Irreproducibility
Last updated on 2025-11-04 | Edit this page
Estimated time: 12 minutes
Overview
Questions
- What are some common reasons for irreproducibility?
Objectives
- Describe a conceptual framework for reproducibility.
- Explain how and why adopting reproducible practices benefits research.
Problems in irreproducible research
From here we will look at some common problems in irreproducible research. We will also discuss ways to overcome these problems. Recall that our definition of reproducible research means that authors provide all data and code to run the analysis again, re-creating the results.
| Methods | Same Data | Different Data |
|---|---|---|
| Same methods | Reproducibility | Replicability |
| Different methods | Robustness | Generalizability |
We will also limit these examples to those that we can examine as analysts. The system of rewards and incentives is one that we must work within for the time being, with an eye toward improving the culture of science. It is not something that we can change in a day however. As analysts we also can’t fix some of the human or technical issues that impact an experiment. We can investigate study design and statistics, however, in data analysis. We will focus on issues with study design, misused methods, and batch effects.
| Factors | Examples |
|---|---|
| Technical | Bad reagents or cell lines, natural variability |
| Study design & statistics | Design flaws, misused methods, batch effects |
| Human | Poor record keeping or sharing, confirmation bias |
| Rewards & incentives | Fraud, paywalls, perverse incentives |
Case 1: The gene set that characterizes early Alzheimer’s disease
K.Q. Watkins and coauthors describe a unique gene set characteristic of early onset Alzheimer’s Disease. The gene expression heatmap from their paper clearly delineates Alzheimer’s patients from a neurotypical control group.
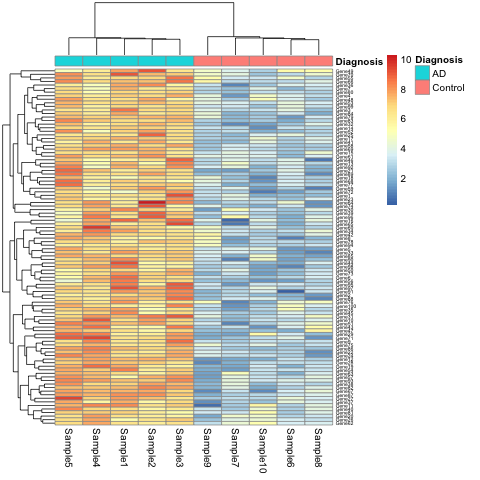
Watkins, K. Q., et al. (2022). A unique gene expression signature characterizes early Alzheimer’s disease. Nature Alzheimer’s, 33(3), 737-753.
Use the R script, the data, and the metadata to reproduce this plot.
Can you find other ways to present the (meta)data in the heatmap? What do alternate ways of presenting the data show you?
This is a simulated study and publication. Any resemblance to real persons or real studies is purely coincidental.
- You can replace
DiagnosiswithBatchin the call thepheatmap.
R
pheatmap(expr_matrix,
annotation_col = metadata["Batch"],
fontsize_row = 5)
This will show the same heatmap, though in this one the legend shows batch number instead of disease state. This is an example of complete confounding between batch and diagnosis. All of the Alzheimer’s samples were run in the first batch and all of the controls in the second. There is no way to disentangle disease state from batch.
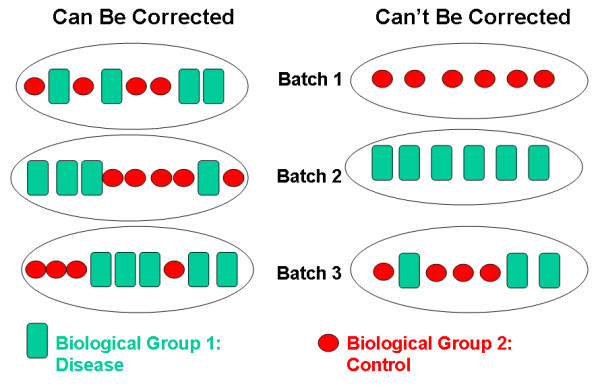
The graphic below shows an example of complete confounding at right
under Can't Be Corrected. Batch 1 contains only controls
and batch 2 only disease samples. There is no method that will be able
to discern the effect of the batch from the effect of disease or
control.
Batch effects are common and can be corrected if samples are
randomized to batch. At left beneath Can Be Corrected,
control and disease samples were randomized to each of three batches.
There can still be batch effects, however, there are methods available
(e.g. ComBat) that can correct for these effects.
Case 2: Hippocampal volume increase in mild cognitive impairment (MCI)
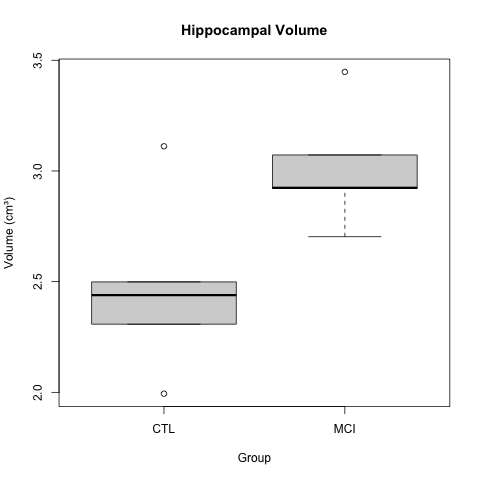
K.Z. Smith and coauthors describe higher mean hippocampal volume in subjects with mild cognitive impairment (MCI). The boxplots below show a clear difference in hippocampal volume between the MCI and control groups.
Smith, K. Z., et al. (2023). Hippocampal volume increase in mild cognitive impairment. Science Progress, 3(14), 37-53.
A t-test gave a p-value of less than 0.05 to reject the null hypothesis of no difference in means between the two groups. The boxplots appear to back up this assertion.
Use the R script and the data to reproduce the boxplot and t-test.
Create a scatterplot of the data by group to get further insight. You can also look at the entire dataset to get a sense of it.
Calculate the effect size between the two groups.
R
# Estimate effect size (Cohen's d for hippocampal volume)
library(effsize)
d_result <- cohen.d(HippocampalVolume ~ Group, data = hippocampus)
print(d_result)
- Use the effect size to calculate statistical power.
R
# Estimate power for hippocampal volume
# Using observed effect size
library(pwr)
power_result <- pwr.t.test(
d = d_result$estimate,
n = n_per_group,
sig.level = 0.05,
type = "two.sample",
alternative = "two.sided"
)
print(power_result)
- What sample size (
n_per_group) would have resulted in 80% statistical power for this experiment?
This is a simulated study and publication. Any resemblance to real persons or real studies is purely coincidental.
R
t_test_result <- t.test(HippocampalVolume ~ Group, data = hippocampus)
print(t_test_result)
boxplot(HippocampalVolume ~ Group, data = hippocampus,
main = "Hippocampal Volume",
ylab = "Volume (cm³)")
R
hippocampus %>% ggplot(aes(Group, HippocampalVolume)) + geom_point()
R
# Estimate effect size (Cohen's d for hippocampal volume)
library(effsize)
d_result <- cohen.d(HippocampalVolume ~ Group, data = hippocampus)
print(d_result)
OUTPUT
Cohen's d
d estimate: -1.558649 (large)
95 percent confidence interval:
lower upper
-3.2238802 0.1065818R
# Estimate power for hippocampal volume
# Using observed effect size
library(pwr)
power_result <- pwr.t.test(
d = d_result$estimate,
n = n_per_group,
sig.level = 0.05,
type = "two.sample",
alternative = "two.sided"
)
print(power_result)
OUTPUT
Two-sample t test power calculation
n = 5
d = 1.558649
sig.level = 0.05
power = 0.581064
alternative = two.sided
NOTE: n is number in *each* groupR
# Update sample size to 8
n_per_group <- 8
# Estimate power for hippocampal volume
# Using observed effect size
power_result <- pwr.t.test(
d = d_result$estimate,
n = n_per_group,
sig.level = 0.05,
type = "two.sample",
alternative = "two.sided"
)
print(power_result)
OUTPUT
Two-sample t test power calculation
n = 8
d = 1.558649
sig.level = 0.05
power = 0.8258431
alternative = two.sided
NOTE: n is number in *each* groupCase 3: A novel biomarker for diagnosis of Alzheimer’s Disease
Your colleague J Mackerel discovered a novel biomarker for Alzheimer’s Disease and provided you with data and an analysis script to review the finding. Run the script to load the data and reproduce the result.
One of the biomarkers had a p-value of less than .05 in a t-test comparing means of the control and Alzheimer’s groups. Your colleague is very excited and ready to go to press with this!
What do you recommend? How would you proceed? Are you convinced of the finding?
P-values are no longer useful to interpret when working with high-dimensional data because we are testing many features at the same time. This is called the multiple comparison or multiple testing problem. When we test many hypotheses simultaneously, a list of p-values can result in many false positives. This example had relatively few features, however, enough t-tests were performed to reasonably expect that a p-value less than .05 would occur.
The solution to the multiple testing problem is correction using a method such as the Bonferroni correction.
- Reproducibility has many definitions.
- We define reproducibility here as using the same data and methods as the original study.
- Adopting reproducible practices strengthens science and makes it more rigorous.
