False Discovery Rate
Last updated on 2025-09-23 | Edit this page
Estimated time: 40 minutes
Overview
Questions
- What are False Discovery Rates, and when are they a concern in data analysis?
- How can you control false discovery rates?
Objectives
- Calculate the false discovery rate.
- Explain the limitations of restricting family wise error rates in a study.
False Discovery Rate
There are many situations for which requiring an FWER of 0.05 does not make sense as it is much too strict. For example, consider the very common exercise of running a preliminary small study to determine a handful of candidate genes. This is referred to as a discovery driven project or experiment. We may be in search of an unknown causative gene and more than willing to perform follow-up studies with many more samples on just the candidates. If we develop a procedure that produces, for example, a list of 10 genes of which 1 or 2 pan out as important, the experiment is a resounding success. With a small sample size, the only way to achieve a FWER \(\leq\) 0.05 is with an empty list of genes. We already saw in the previous section that despite 1,000 diets being effective, we ended up with a list with just 2. Change the sample size to 6 and you very likely get 0:
R
set.seed(1)
population <- unlist(read.csv(file = "./data/femaleControlsPopulation.csv"))
m <- 10000
p0 <- 0.90
m0 <- m*p0
m1 <- m-m0
nullHypothesis <- c( rep(TRUE,m0), rep(FALSE,m1))
delta <- 3
pvals <- sapply(1:m, function(i){
control <- sample(population, 6)
treatment <- sample(population, 6)
if(!nullHypothesis[i]) treatment <- treatment + delta
t.test(treatment, control)$p.value
})
sum(pvals < 0.05/10000)
OUTPUT
[1] 0By requiring a FWER \(\leq\) 0.05, we are practically assuring 0 power (sensitivity). In many applications, this specificity requirement is over-kill. A widely used alternative to the FWER is the false discovery rate (FDR). The idea behind FDR is to focus on the random variable \(Q \equiv V/R\) with \(Q=0\) when \(R=0\) and \(V=0\). Note that \(R=0\) (nothing called significant) implies \(V=0\) (no false positives). So \(Q\) is a random variable that can take values between 0 and 1 and we can define a rate by considering the average of \(Q\). To better understand this concept here, we compute \(Q\) for the procedure: call everything p-value < 0.05 significant.
Discussion
Refer to the confusion matrix below showing false positive, false
negative, and false discovery rates, along with specificity and
sensitivity. Turn to a partner and explain the following:
How is the false discovery rate related to Type I error and false
positive rates?
How is sensitivity related to the false discovery rate?
When you are finished discussing, share with the group in the
collaborative document.
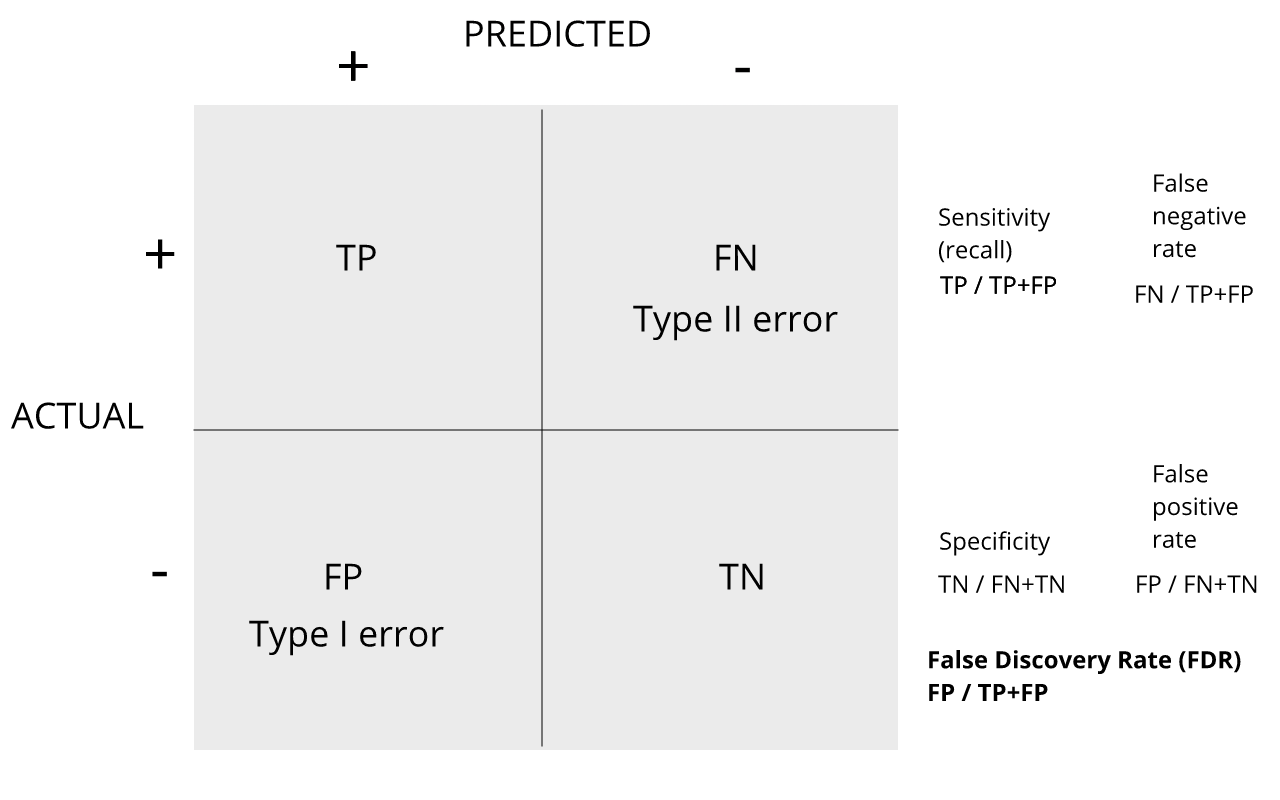
For more on this, see Classification evaluation by J. Lever, M. Krzywinski and N. Altman in Nature Methods 13, 603-604 (2016).
Vectorizing code
Before running the simulation, we are going to vectorize the
code. This means that instead of using sapply to run
m tests, we will create a matrix with all data in one call
to sample. This code runs several times faster than the code above,
which is necessary here due to the fact that we will be generating
several simulations. Understanding this chunk of code and how it is
equivalent to the code above using sapply will take a you
long way in helping you code efficiently in R.
R
library(genefilter) ##rowttests is here
alpha <- 0.05
N <- 12
set.seed(1)
##Define groups to be used with rowttests
g <- factor( c(rep(0, N), rep(1, N)) )
B <- 1000 ##number of simulations
Qs <- replicate(B, {
##matrix with control data (rows are tests, columns are mice)
controls <- matrix(sample(population, N*m, replace=TRUE), nrow=m)
##matrix with control data (rows are tests, columns are mice)
treatments <- matrix(sample(population, N*m, replace=TRUE), nrow=m)
##add effect to 10% of them
treatments[which(!nullHypothesis),] <- treatments[which(!nullHypothesis),] + delta
##combine to form one matrix
dat <- cbind(controls, treatments)
calls <- rowttests(dat, g)$p.value < alpha
R=sum(calls)
Q=ifelse(R > 0, sum(nullHypothesis & calls)/R, 0)
return(Q)
})
Controlling FDR
The code above is a Monte Carlo simulation that generates 10,000 experiments 1,000 times, each time saving the observed \(Q\). Here is a histogram of these values:
R
library(rafalib)
mypar(1, 1)
hist(Qs) ##Q is a random variable, this is its distribution
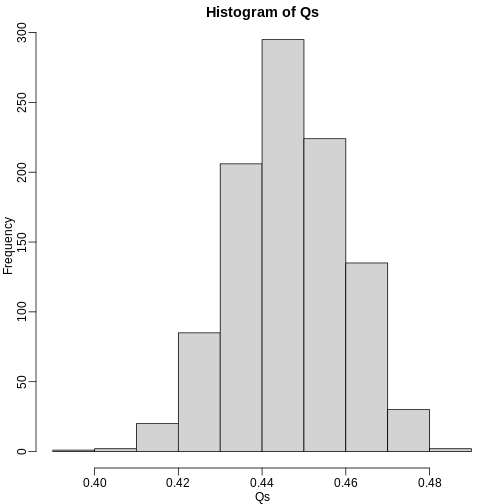
The FDR is the average value of \(Q\)
R
FDR=mean(Qs)
print(FDR)
OUTPUT
[1] 0.4465395The FDR is relatively high here. This is because for 90% of the tests, the null hypotheses is true. This implies that with a 0.05 p-value cut-off, out of 100 tests we incorrectly call between 4 and 5 significant on average. This combined with the fact that we don’t “catch” all the cases where the alternative is true, gives us a relatively high FDR. So how can we control this? What if we want lower FDR, say 5%?
To visually see why the FDR is high, we can make a histogram of the
p-values. We use a higher value of m to have more data from
the histogram. We draw a horizontal line representing the uniform
distribution one gets for the m0 cases for which the null
is true.
R
set.seed(1)
controls <- matrix(sample(population, N*m, replace=TRUE), nrow=m)
treatments <- matrix(sample(population, N*m, replace=TRUE), nrow=m)
treatments[which(!nullHypothesis),] <- treatments[which(!nullHypothesis),] + delta
dat <- cbind(controls, treatments)
pvals <- rowttests(dat, g)$p.value
h <- hist(pvals, breaks=seq(0,1,0.05))
polygon(c(0,0.05,0.05,0), c(0,0,h$counts[1],h$counts[1]), col="lightsteelblue1")
abline(h=m0/20)
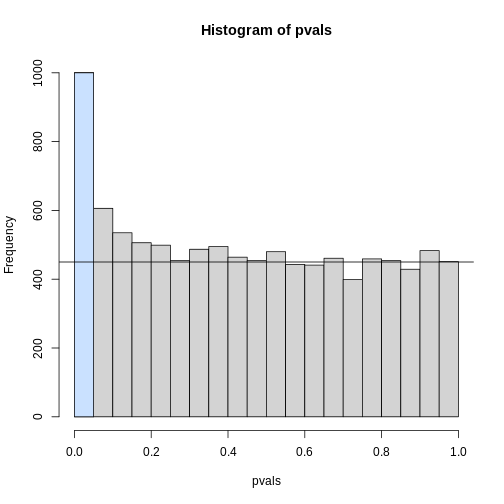
The first bar (grey) on the left represents cases with p-values smaller than 0.05. From the horizontal line we can infer that about 1/2 are false positives. This is in agreement with an FDR of 0.50. If we look at the bar for 0.01, we can see a lower FDR, as expected, but would call fewer features significant.
R
h <- hist(pvals,breaks=seq(0,1,0.01))
polygon(c(0,0.01,0.01,0),c(0,0,h$counts[1],h$counts[1]),col="lightsteelblue1")
abline(h=m0/100)
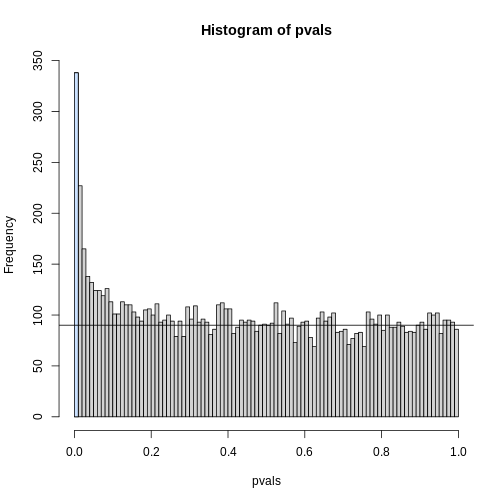
As we consider a lower and lower p-value cut-off, the number of features detected decreases (loss of sensitivity), but our FDR also decreases (gain of specificity). So how do we decide on this cut-off? One approach is to set a desired FDR level \(\alpha\), and then develop procedures that control the error rate: FDR \(\leq \alpha\).
Benjamini-Hochberg (Advanced)
We want to construct a procedure that guarantees the FDR to be below a certain level \(\alpha\). For any given \(\alpha\), the Benjamini-Hochberg (1995) procedure is very practical because it simply requires that we are able to compute p-values for each of the individual tests and this permits a procedure to be defined.
For this procedure, order the p-values in increasing order: \(p_{(1)},\dots,p_{(m)}\). Then define \(k\) to be the largest \(i\) for which
\[p_{(i)} \leq \frac{i}{m}\alpha\]
The procedure is to reject tests with p-values smaller or equal to \(p_{(k)}\). Here is an example of how we would select the \(k\) with code using the p-values computed above:
R
alpha <- 0.05
i = seq(along=pvals)
mypar(1,2)
plot(i,sort(pvals))
abline(0,i/m*alpha)
##close-up
plot(i[1:15],sort(pvals)[1:15],main="Close-up")
abline(0,i/m*alpha)
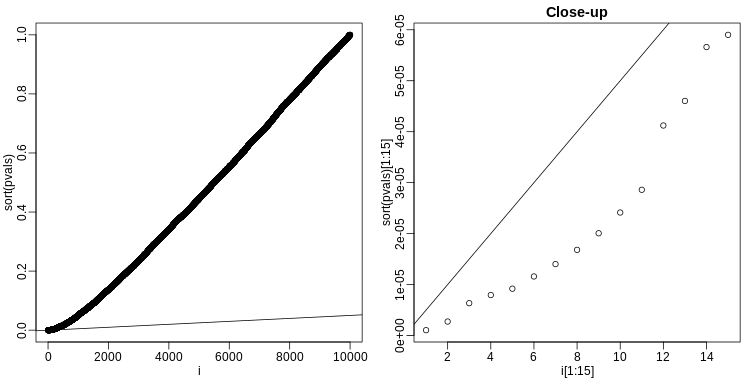
R
k <- max( which( sort(pvals) < i/m*alpha) )
cutoff <- sort(pvals)[k]
cat("k =",k,"p-value cutoff=",cutoff)
OUTPUT
k = 24 p-value cutoff= 0.0001197627We can show mathematically that this procedure has FDR lower than 5%. Please see Benjamini-Hochberg (1995) for details. An important outcome is that we now have selected 11 tests instead of just 2. If we are willing to set an FDR of 50% (this means we expect at least 1/2 our genes to be hits), then this list grows to 1063. The FWER does not provide this flexibility since any list of substantial size will result in an FWER of 1.
Keep in mind that we don’t have to run the complicated code above as
we have functions to do this. For example, using the p-values
pvals computed above, we simply type the following:
R
fdr <- p.adjust(pvals, method="fdr")
mypar(1,1)
plot(pvals,fdr,log="xy")
abline(h=alpha,v=cutoff) ##cutoff was computed above
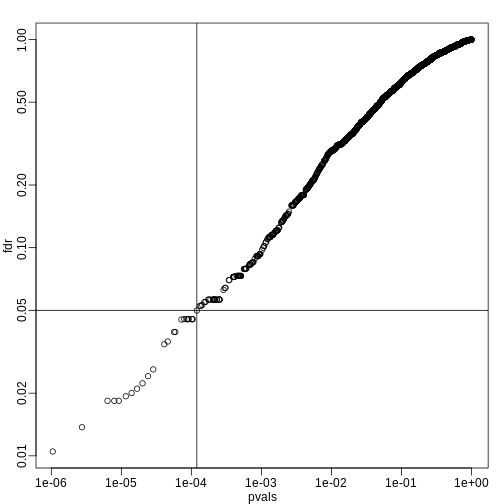
We can run a Monte-Carlo simulation to confirm that the FDR is in fact lower than .05. We compute all p-values first, and then use these to decide which get called.
R
alpha <- 0.05
B <- 1000 ##number of simulations. We should increase for more precision
res <- replicate(B,{
controls <- matrix(sample(population, N*m, replace=TRUE),nrow=m)
treatments <- matrix(sample(population, N*m, replace=TRUE),nrow=m)
treatments[which(!nullHypothesis),]<-treatments[which(!nullHypothesis),]+delta
dat <- cbind(controls,treatments)
pvals <- rowttests(dat,g)$p.value
##then the FDR
calls <- p.adjust(pvals,method="fdr") < alpha
R=sum(calls)
Q=ifelse(R>0,sum(nullHypothesis & calls)/R,0)
return(c(R,Q))
})
Qs <- res[2,]
mypar(1,1)
hist(Qs) ##Q is a random variable, this is its distribution
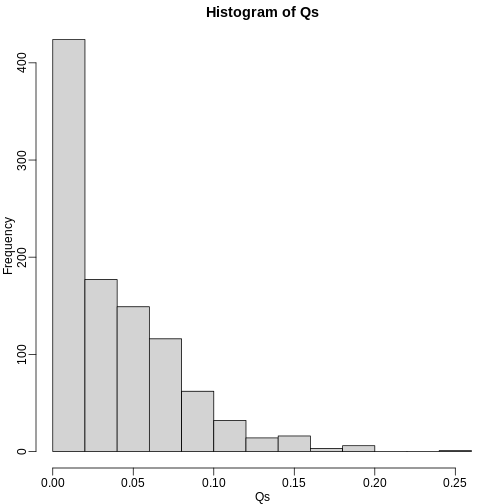
R
FDR=mean(Qs)
print(FDR)
OUTPUT
[1] 0.03641142The FDR is lower than 0.05. This is to be expected because we need to be conservative to ensure the FDR \(\leq\) 0.05 for any value of \(m_0\), such as for the extreme case where every hypothesis tested is null: \(m=m_0\). If you re-do the simulation above for this case, you will find that the FDR increases.
We should also note that in …
R
Rs <- res[1,]
mean(Rs==0) * 100
OUTPUT
[1] 1.3… percent of the simulations, we did not call any genes significant.
Finally, note that the p.adjust function has several
options for error rate controlling procedures:
R
p.adjust.methods
OUTPUT
[1] "holm" "hochberg" "hommel" "bonferroni" "BH"
[6] "BY" "fdr" "none" It is important to remember that these options offer not just different approaches to estimating error rates, but also that different error rates are estimated: namely FWER and FDR. This is an important distinction. More information is available from:
R
?p.adjust
In summary, requiring that FDR \(\leq\) 0.05 is a much more lenient requirement FWER \(\leq\) 0.05. Although we will end up with more false positives, FDR gives us much more power. This makes it particularly appropriate for discovery phase experiments where we may accept FDR levels much higher than 0.05.
- Restricting FWER too much can cause researchers to reject the null hypothesis when it’s actually true. This is especially likely in the small samples used in discovery phase experiments.
- The Benjamini-Hochberg correction controls FDR by guaranteeing it to be below a desired alpha level.
- FDR is a more liberal correction than Bonferroni. While it generates more false positives, it also provides more statistical power.
